The U.S. Food and Drug Administration’s Center for Veterinary Medicine (FDA-CVM) is asking veterinarians and animal care retailers to educate people on the harms of using the animal drug ivermectin to try to treat or prevent COVID-19.
According to an August 30, 2021 FDA-CVM letter, people are buying various highly concentrated animal ivermectin drug formulations including “pour-on,” injectable, paste, and “drench” that are intended for horses, cattle, and sheep. Poison control centers nationwide are noticing a surge in people experiencing adverse health effects and becoming severely sick after taking animal ivermectin.
Though some animal drugs have the same active ingredient as an approved human drug, animal drugs have not been evaluated for human safety or effectiveness. Using veterinary drugs for human conditions can be dangerous, as the drug may be completely ineffective or could worsen the illness and/or lead to serious, potentially life-threatening health complications. Additionally, humans should not take drugs approved for veterinary use, “for research only,” or otherwise not for human consumption.
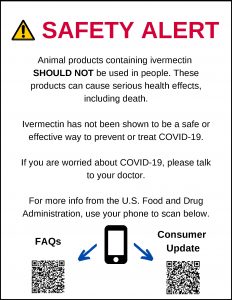 To help veterinarians and retailers inform the public, the CVM has created a downloadable sign to pass out or post at a place of business that helps explain the perils of taking animal ivermectin for human use.
To help veterinarians and retailers inform the public, the CVM has created a downloadable sign to pass out or post at a place of business that helps explain the perils of taking animal ivermectin for human use.
In certain parts of the country, there has been limited availability of animal ivermectin products. If you are a veterinarian or animal caretaker and are struggling to obtain this drug for animal use, FDA-CVM suggests emailing AnimalDrugShortages@fda.hhs.gov.
To help protect public health, FDA-CVM also asks you to report any animal drug advertising/animal ivermectin products with statements about preventing or treating COVID-19 by emailing FDA-COVID-19-Fraudulent-Products@fda.hhs.gov or calling 1-888-InfoFDA (1-888-463-6332).

